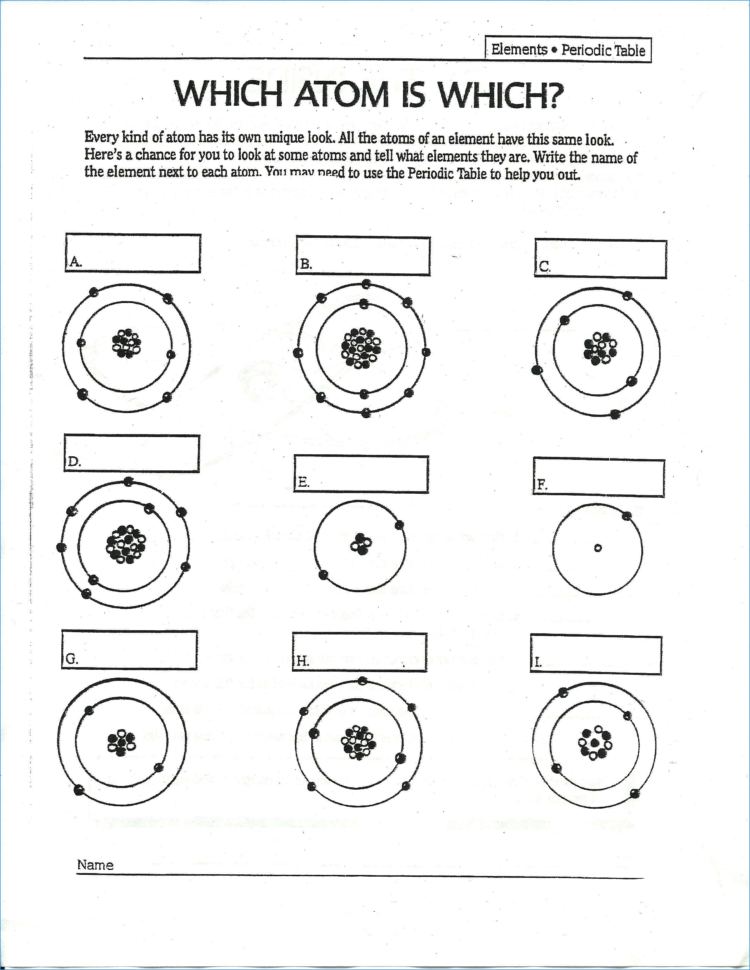
Lewis Dot Diagram Periodic Table Worksheet. The number of valence electrons can also be. determined by simply determining the group number on the periodic table. The number of valence electrons can also be determined by simply determining the group number on the periodic table.
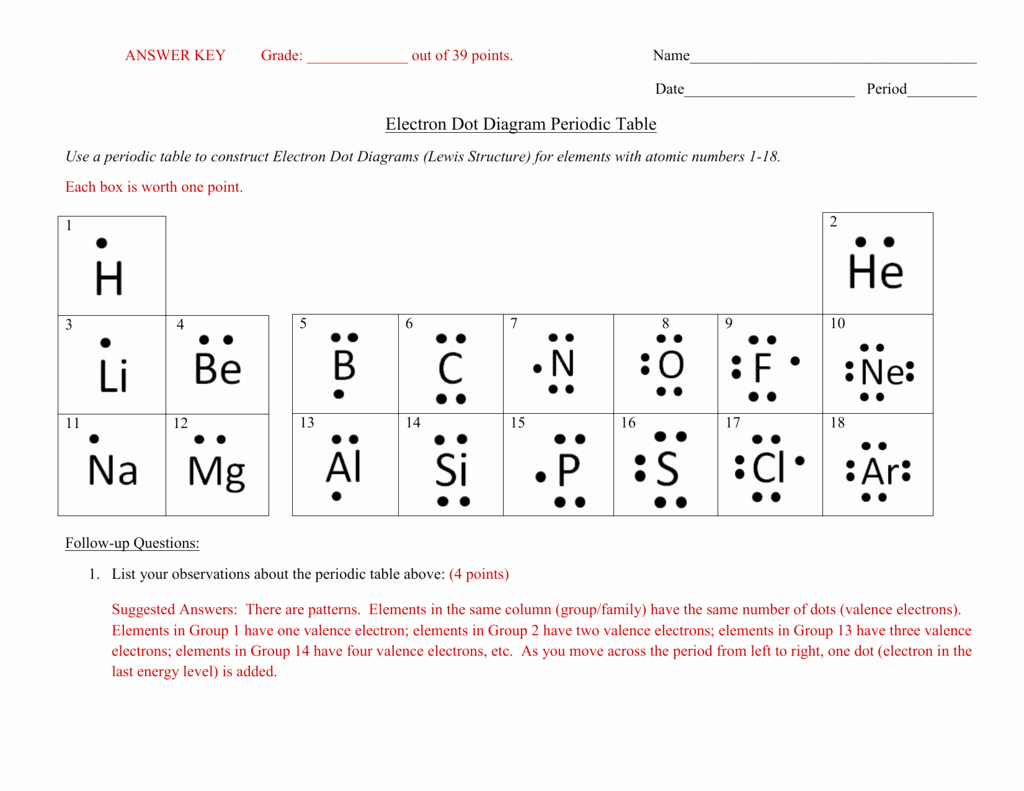
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol.
A notation showing the valence electrons surrounding the atomic symbol.
Electron Dot Structure or Lewis Dot Diagram (Gilbert Lewis). Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the outermost energy level of an atom. Drawing Lewis dot structures (also known as Lewis structures or Lewis diagrams) can be confusing, particularly for a beginning chemistry student.